Head and Neck Lymphedema Solutions
Explore the Flexitouch® Plus system, a head and neck lymphedema pneumatic compression solution that can be worn at home to relieve swelling and related symptoms, such as difficulty in swallowing and breathing and pain. Incorporating the Flexitouch Plus into your daily lymphedema management routine can help you maintain your quality of life and find relief.
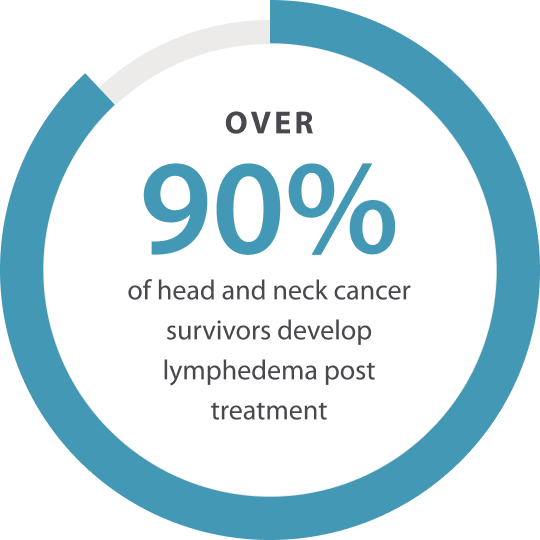
Many head and neck cancer survivors are faced with an additional challenge: lymphedema.
Head and neck cancer treatments often lead to lymphedema – a chronic swelling condition that may be caused by damage to the lymphatic system. The swelling can be both internal and external and cause pain, reduced range of motion and difficulty swallowing, speaking and breathing.
Lymphedema can be debilitating and make it hard for survivors of head and neck cancer to start enjoying life again.
As with any chronic, progressive condition, early intervention and practical tools for daily management produce the best results.
Head and Neck Lymphedema Treatment
Flexitouch Plus provides a comfortable, effective, at-home treatment designed to help head and neck lymphedema patients get back to their lives.
The Flexitouch Plus system is clinically proven to stimulate the lymphatic system and to treat head and neck lymphedema.
The head and neck garment set is easy to use and features the clinically proven Flexitouch technology that tens of thousands of lymphedema patients rely on every day to effectively treat their extremities.
Flexitouch Plus helps reduce swelling and relieve symptoms of head and neck lymphedema with effective treatments that are:
Consistent: Unlike manual lymphatic drainage (MLD), effective head and neck lymphedema treatment doesn’t depend on patient technique or ability. doesn’t depend on patient technique or ability. Repeatable results and high patient satisfaction encourage ongoing use and better outcomes.
Comfortable: Head and neck compression garments are made of soft, comfortable fabric while the dynamic pressure feels like a soothing massage.
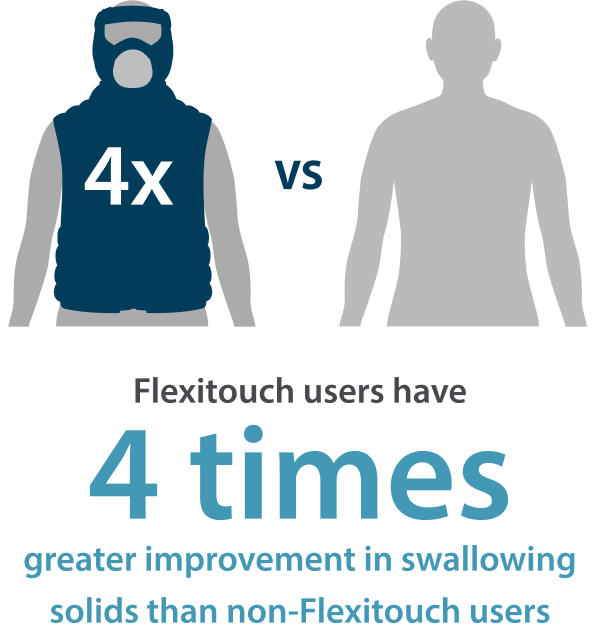
Clinical evidence shows Flexitouch combined with self-management is proven to work better than self-management alone, providing better outcomes for patients.
Convenient: The patient-friendly design of the head and chest garments is created for ease of use.
Discover
Flexitouch® Plus
for Head and Neck
Empowering patients to self-manage their head and neck lymphedema from home for improved outcomes and a better quality of life.
Tactile Medical is a leader among clinical studies and the Flexitouch system has been studied clinically in more than 1,800 patients. The Flexitouch system is clinically proven to improve clinical outcomes and reduce healthcare costs, stimulate lymphatic function, and significantly reduce edema and limb volume.
The Flexitouch Plus helps to improve the lives of patients who have struggled with the pain, swallowing, speaking and breathing difficulties brought on by head and neck lymphedema.
Upcoming webinars
Register for our upcoming live webinars or take a look at past webinars and other resources for the latest research
Need help now? We’re here for you.
Call us at 1.833.3TACTILE (1-833-382-2845)
Head and Neck Lymphedema FAQs
What is head and neck lymphedema?
Head and neck lymphedema is a chronic, progressive condition that’s caused by a disruption to the lymphatic system, leading to a buildup of fluid in the tissues of the head and neck. Head and neck lymphedema can be categorized as primary or secondary. Primary head and neck lymphedema can be hereditary and passed down from a parent, present at birth (congenital), or spontaneous in nature. Secondary head and neck lymphedema is more common and is the result of damage or restrain to the lymphatic system, such as direct trauma from an injury, cancer, or cancer treatment.
What are the symptoms of head and neck lymphedema?
The symptoms of head and neck lymphedema can vary from person to person but can occur internally in locations like the throat, mouth, tongue, and voice box or externally in the face and neck. However, whether you’re living with facial lymphedema or neck lymphedema, the common symptoms include:
- Swelling in the face, neck, or throat
- Difficulty swallowing, eating, speaking, or breathing
- Congestion
- Changes in skin texture
- Decreased range of motion, stiffness, or pain in the neck and shoulders
- Pain and swelling that feels hard when touched
- Changes in vision or hearing
What causes head and neck lymphedema?
In cases of primary head and neck lymphedema, the cause can be genetic, present at birth, or spontaneous in nature. However, secondary head and neck lymphedema is more common and can have several different causes. Some of the most common causes of lymphedema in the head and neck and lymphedema risk factors to be aware of include:
- Cancer: If a cancer tumor disrupts the flow of lymph fluid in the head and neck, lymphedema can occur.
- Cancer treatment and radiation therapy: Removal of a lymph node or vessel, along with scarring from surgical procedures and damage of nodes and vessels from radiation, can disrupt the flow of fluid in the lymphatics, leading to lymphedema.
While cancer and its treatments, including cancer surgery and/or radiation therapy, are the most common causes of head and neck lymphedema, other potential causes include trauma/injury to the lymphatics around the head and neck, infections like cellulitis, and vascular diseases.
How do you treat head and neck lymphedema?
Lymphedema treatment involves a personalized approach depending on the cause and symptoms of your head and neck lymphedema. For many lymphedema patients, a multipronged approach, including head and neck lymphedema compression garments, exercise, and healthy lifestyle modifications, will be recommended by your doctor or certified lymphedema therapist. Below are some of the components of a head and neck lymphedema treatment plan:
- Complete decongestive therapy (CDT): CDT combines several treatment methods to stimulate the lymphatics and relieve pain, swelling, and related symptoms. It starts with in-clinic treatment provided by a lymphedema therapist and is followed by self-management at home. The treatment includes exercise, manual lymphatic drainage, skin care, and compression.
- Manual lymphatic drainage (MLD): MLD is a specialized massage technique that stimulates the flow of fluid throughout the lymphatics. Pneumatic compression devices like the Flexitouch Plus system mimic MLD and are often recommended by doctors for at-home maintenance.
- Compression: To reduce and maintain the reduction of swelling, compression garments for head and neck lymphedema are recommended
- Exercise: Head and neck lymphedema exercises, such as stretching, head tilts, shoulder shrugs, and yoga, help promote lymphatic drainage and maintain a healthy weight.
- Skin care: Proper skin care for lymphedema helps to prevent infections and complications that can exacerbate swelling and other symptoms.
Following the elements of CDT, including wearing compression garments for lymphedema, manual lymphatic drainage for the head and neck, exercise, and a skin care routine, can help provide relief from lymphedema and related symptoms. Contact Tactile Medical today to learn how our head and neck lymphedema therapy can help.
1. Ridner, S.H., et al., A Prospective Study of the Lymphedema and Fibrosis Continuum in Patients with Head and Neck Cancer. Lymphat Res Biol, 2016. 14(4): p. 198-205.
2. Ridner, S.H., Dietrich, M.S., Deng, J. et al. Advanced Pneumatic Compression for Treatment of Lymphedema of the Head and Neck: A Randomized Wait-List Controlled Trial. Support Care Cancer (2020). https://doi.org/10.1007/s00520-020-05540-8
3. Karaca-Mandic P, Hirsch AT, Rockson SG, et al. The Cutaneous, Net Clinical, and Health Economic Benefits of Advanced Pneumatic Compression Devices in Patients With Lymphedema. JAMA Dermatol. 2015;151(11):1187–1193
4. Muluk SC, Hirsch AT, Tafe EC. Pneumatic Compression Device Treatment of Lower Extremity Lymphedema Elicits Improved Limb Volume and Patient-Reported Outcomes. EJVES. 2013; Vol. 46(4): 480–487.
5. Adams, KE, Rasmussen JC, Darne C, et al. Direct Evidence of Lymphatic Function Improvement After Advanced Pneumatic Compression Device Treatment of Lymphedema. Biomedical Optics Express. 2010; Vol. 1(1): 114–125.


