Flexitouch® Plus User Guide for Upper Body
The Flexitouch Plus system is designed for at-home treatment of lymphedema, chronic edema, chronic venous insufficiency (CVI) and chronic wounds. This guide provides the information needed to set up and use your Flexitouch Plus system.
Helpful Videos
Flexitouch Plus Program Options for Upper Body
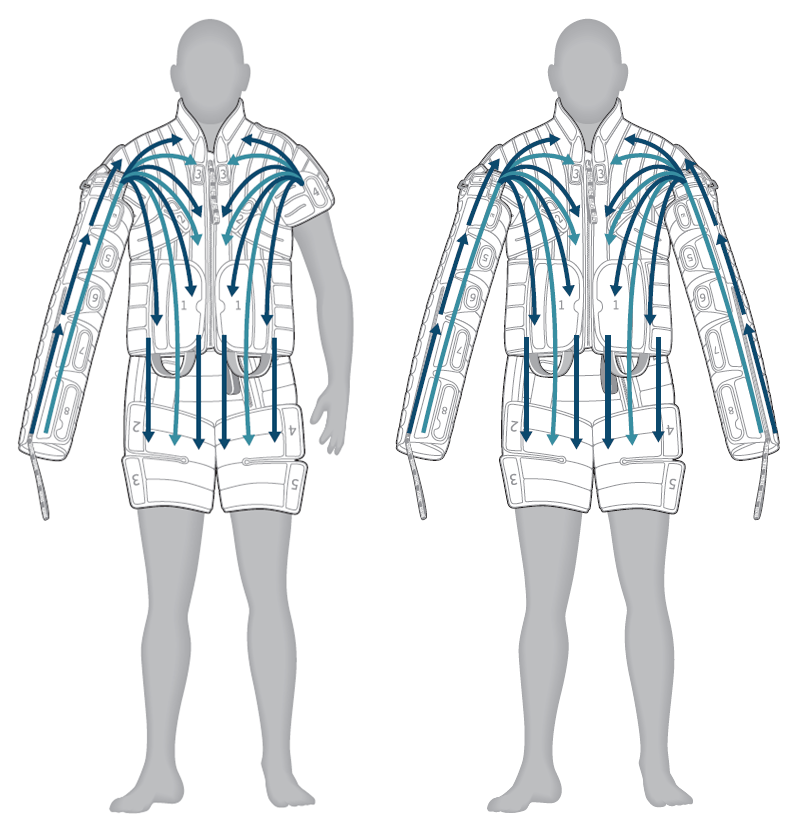
FULL ARM AND CORE TREATMENT: PROGRAM U1/CU1
This program provides distal to proximal treatment preceded by proximal clearing of lymphatic fluid in the trunk, chest, shoulder, biceps, forearm and hand. Enhanced programming applies treatment that extends past the axilla area into the chest and trunk, providing comprehensive treatment to patients when clinically appropriate.
Treatment time:
60 minutes
How it works:
- Trunk: directs fluid from the waist toward the top of the thighs; cycle repeats
- Chest: directs fluid from the shoulder toward the waist; cycle repeats
- Biceps: directs fluid from the elbow toward the shoulder; cycle repeats
- Forearm: directs fluid from the wrist toward the elbow; cycle repeats
- Hand: directs fluid from the fingers toward the wrist; cycle repeats
- Full arm, shoulder, chest and trunk: directs fluid from the fingers to the top of the thigh in one continuous motion; cycle repeats
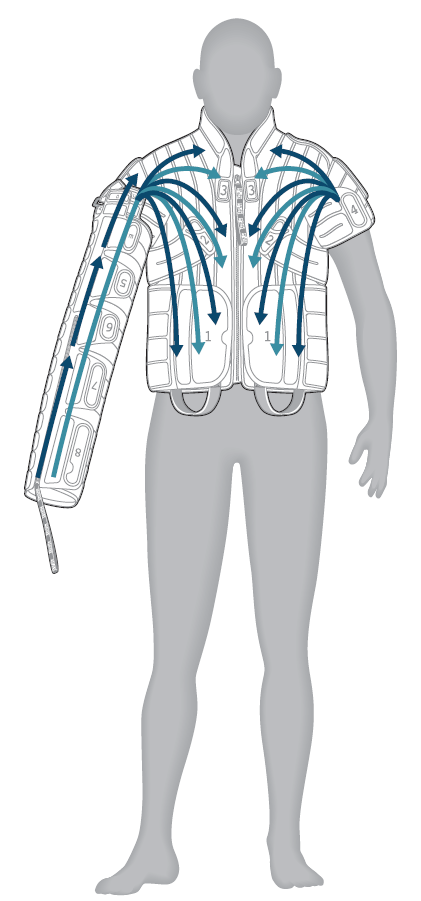
ARM-SHOULDER TREATMENT: PROGRAM U4/CU4
This treatment program provides distal to proximal treatment preceded by proximal clearing of lymphatic fluid in the chest, shoulder, biceps, forearm and hand. The chambers sequentially inflate and deflate to stimulate and decongest this region of the lymphatic system. Only one chamber is fully inflated at any point in time.
Treatment time:
50 minutes
How it works:
- Chest: directs fluid from the shoulder toward the waist; cycle repeats
- Biceps: directs fluid from the elbow toward the shoulder; cycle repeats
- Forearm: directs fluid from the wrist toward the elbow; cycle repeats
- Hand: directs fluid from the fingers toward the wrist; cycle repeats
- Full arm, shoulder and chest: directs fluid proximally from the fingers up to the shoulder, then to the top of the waist in one continuous motion; cycle repeats
INDICATIONS FOR USE:
The Flexitouch Plus system and garments for legs, arms, trunk and chest are intended for use by medical professionals and patients who are under medical supervision, for the treatment of many conditions such as:
- Lymphedema
- Primary Lymphedema
- Post mastectomy edema
- Phlebolymphedema
- Lipedema
- Edema following trauma and sports injuries
- Post immobilization edema
- Venous insufficiency
- Reducing wound healing time
- Treatment and assistance in healing stasis dermatitis, venous stasis ulcers, or arterial and diabetic leg ulcer
CONTRAINDICATIONS:
The Flexitouch Plus system should not be used if you have one or more of the following conditions:
- Heart failure (acute pulmonary edema, decompensated acute heart failure)
- Acute venous disease (acute thrombophlebitis, acute deep venous thrombosis, acute pulmonary embolism)
- Severe peripheral artery disease (critical limb ischemia including ischemic rest pain, arterial wounds or gangrene)
- Active skin or limb infection/inflammatory disease (acute cellulitis, other uncontrolled skin or untreated inflammatory skin disease)
- Any circumstance where increased lymphatic or venous return is undesirable
- During pregnancy (applies only to the Flexitouch Plus trunk accessory)
CLINICAL DOCUMENTATION GUIDELINES FOR FLEXITOUCH PLUS THERAPY:
Most health plans have specific policies that outline medical necessity requirements. Medical records may require documentation that addresses the following:
- 4 weeks of conservative therapy (compression, elevation, and exercise)
- If clinical symptoms persist despite conservative care, what are they? (i.e., continued swelling, compromised skin integrity, pain, recurrent cellulitis)
- If a basic pneumatic compression device is not appropriate for the patient, what is the clinical rationale? (i.e., chest/trunk swelling)
TRAVELING WITH YOUR FLEXITOUCH PLUS SYSTEM:
Patients may travel with any device that is medically necessary and can go through the x-ray screening process. Tactile Medical recommends patients traveling with their device do the following:
- Carry on their controller and power cord; patients may pack any garments/sleeves in their checked luggage
- Bring their user guide and prescription card with them to better help explain what the device does to a TSA agent
- Take the controller out of the carry on, as is done with a laptop
- Be prepared to turn the device on and show a TSA agent how the device works, if asked to do so
- For more information, visit our travel page at https://tactilemedical.com/travel/
- Websites such as www.tsa.gov, or the equivalent agency in your destination country may be able to give your more information
ADDITIONAL HELPFUL INFORMATION:
- The device will operate from 100 – 240 volts, 50/60 Hz, working nearly anywhere in the world without the need for a transformer or voltage converter. If traveling outside the US, a plug adapter may be necessary, and can be purchased at a retail store. Tactile Medical does not supply plug adapters.
- Websites such as www.tsa.gov, or the equivalent agency in your destination country may be able to give your more information.
- The following FDA website link show our products have been cleared to market in the US: https://www.accessdata.fda.gov/cdrh_docs/pdf17/K170216.pdf
- If you have any additional questions, please contact Tactile Medical at 1.833.3TACTILE (1-833-382-2845).


