Flexitouch® Plus User Guide for Head and Neck
The Flexitouch Plus system is designed for at-home treatment of lymphedema, chronic edema, chronic venous insufficiency (CVI) and chronic wounds. This guide provides the information needed to set up and use your Flexitouch Plus system.
Helpful Videos
FLEXITOUCH PLUS PROGRAM FOR HEAD, NECK AND CHEST
HEAD, NECK AND CHEST TREATMENT: PROGRAM H1/CH1
This program provides therapy to the head, neck and chest areas. The therapy begins with stimulation to adjacent healthy lymph node groups in the chest and axilla to allow for optimal lymph drainage of the head, neck and face.
Treatment time:
32 minutes
How it works:
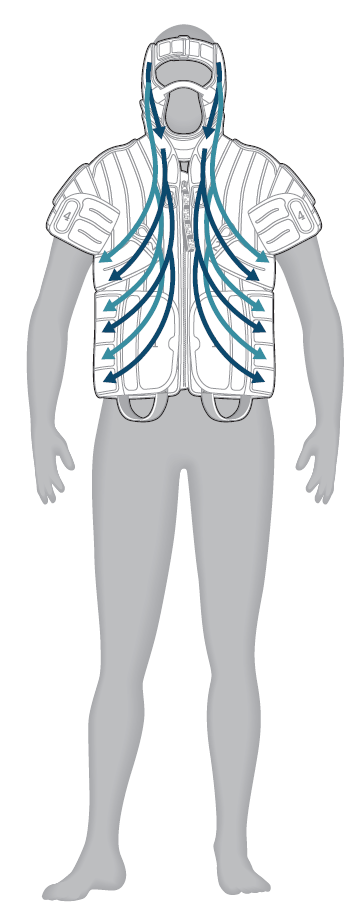
- Chest and neck: directs fluid from the neck and the chest, toward the underarm (axilla); cycle repeats
- Head: directs fluid from head toward the neck; cycle repeats
- Head, neck and chest: directs fluid from head toward the underarm area in one continuous motion; cycle repeats
INDICATIONS FOR USE:
The Flexitouch Plus system and garments for legs, arms, trunk and chest are intended for use by medical professionals and patients who are under medical supervision, for the treatment of many conditions such as:
- Lymphedema
- Primary Lymphedema
- Post mastectomy edema
- Phlebolymphedema
- Lipedema
- Edema following trauma and sports injuries
- Post immobilization edema
- Venous insufficiency
- Reducing wound healing time
- Treatment and assistance in healing stasis dermatitis, venous stasis ulcers, or arterial and diabetic leg ulcers
The Flexitouch Plus system and garments for the head and neck are intended for use by medical professionals and patients who are under medical supervision for the treatment of head and neck lymphedema.
CONTRAINDICATIONS:
The Flexitouch Plus system should not be used if you have one or more of the following conditions:
- Heart failure (acute pulmonary edema, decompensated acute heart failure)
- Acute venous disease (acute thrombophlebitis, acute deep venous thrombosis, acute pulmonary embolism)
- Severe peripheral artery disease (critical limb ischemia including ischemic rest pain, arterial wounds or gangrene)
- Active skin or limb infection/inflammatory disease (acute cellulitis, other uncontrolled skin or untreated inflammatory skin disease)
- Any circumstance where increased lymphatic or venous return is undesirable
- During pregnancy (applies only to the Flexitouch Plus trunk accessory)
The head garment and vest for head and neck for the Flexitouch Plus system should not be used if you have one or more of the following conditions:
- Uncontrolled hyperthyroidism or parathyroidism (for which an endocrinologist recommends against neck compression)
- Carotid sinus hypersensitivity syndrome
- Symptomatic carotid artery disease, as manifested by a recent transient ischemic attack (within 30 days), ischemic stroke or amaurosis fugax (monocular visual ischemic symptoms or blindness)
- Symptomatic bradycardia in the absence of a pacemaker
- Internal jugular venous thrombosis (within three months)
- Increased intracranial pressure or other contraindication to internal or external jugular venous compression
- Acute radiation dermatitis, unhealed surgical scar, unhealed or open wound(s), surgical flap less than six to eight week post-operative
- Facial or head and neck dermal metastasis
- Acute facial infection (e.g., facial or parotid gland abscess)
- Any condition in which increased venous and lymphatic return is undesirable
TRAVELING WITH YOUR FLEXITOUCH PLUS SYSTEM:
Patients may travel with any device that is medically necessary and can go through the x-ray screening process. Tactile Medical recommends patients traveling with their device do the following:
- Carry on their controller and power cord; patients may pack any garments/sleeves in their checked luggage
- Bring their user guide and prescription card with them to better help explain what the device does to a TSA agent
- Take the controller out of the carry on, as is done with a laptop
- Be prepared to turn the device on and show a TSA agent how the device works, if asked to do so
- For more information, visit our travel page at https://tactilemedical.com/travel/
- Websites such as www.tsa.gov, or the equivalent agency in your destination country may be able to give your more information
ADDITIONAL HELPFUL INFORMATION:
- The device will operate from 100 – 240 volts, 50/60 Hz, working nearly anywhere in the world without the need for a transformer or voltage converter. If traveling outside the US, a plug adapter may be necessary, and can be purchased at a retail store. Tactile Medical does not supply plug adapters.
- Websites such as www.tsa.gov, or the equivalent agency in your destination country may be able to give your more information.
- The following FDA website link show our products have been cleared to market in the US: https://www.accessdata.fda.gov/cdrh_docs/pdf17/K170216.pdf
- If you have any additional questions, please contact Tactile Medical at 1.833.3TACTILE (1-833-382-2845).


